15+ Le Chatelier Principle Pdf
KCl is used as a fertilizer in medicine. First in situ CO 2 removal allows the use of reducing gas feedstocks that are ranked lower in the value chain than natural gasfor example biogas containing up to.

Chemistry For The Ib Diploma Second Edition By Cambridge University Press Education Issuu
Ionic equilibrium- ionization of acids and bases strong and weak electrolytes degree.

. Biology syllabus of class 11th. Web Oilfield scale inhibition is the process of preventing the formation of scale from blocking or hindering fluid flow through pipelines valves and pumps used in oil production and processing. The driving power for pitting corrosion is the depassivation of a small area which becomes anodic oxidation reaction while an unknown but potentially vast area becomes cathodic reduction reaction leading to very localized.
Web Chemical equations are used to graphically illustrate chemical reactions. Web In the study of combustion the adiabatic flame temperature is the temperature reached by a flame under ideal conditions. They are separated by an arrow which indicates the direction and type of the reaction.
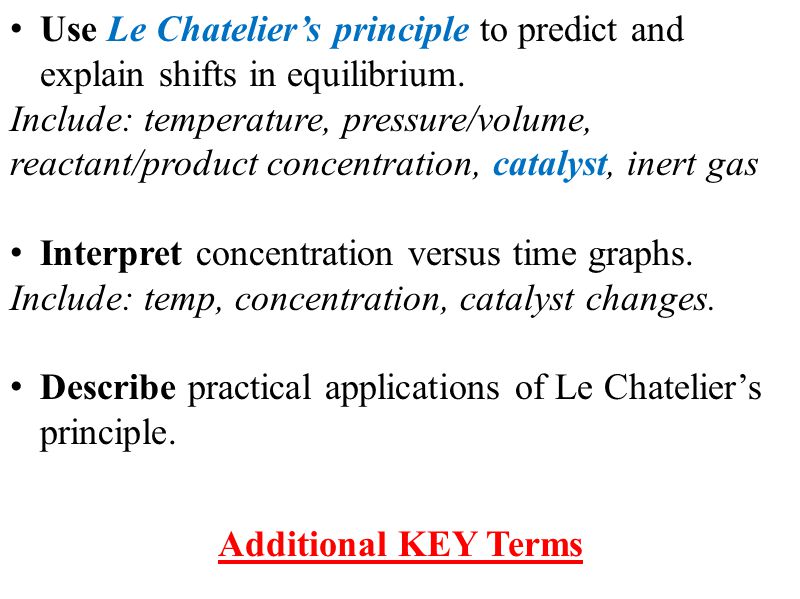
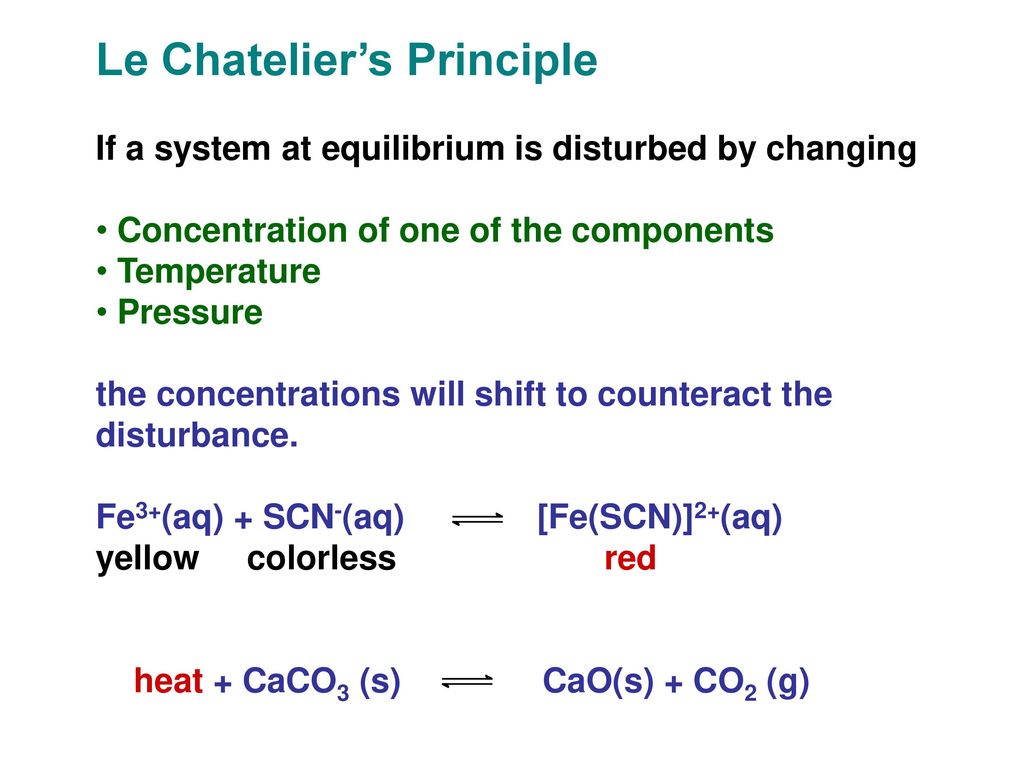
Web The dissolution of calcium hydroxide in water is also an exothermic process ΔH 0 and obeys the van t Hoff equation and Le Chateliers principle. Chemistry Perfect Score X A Plus Module 2013 1 MODUL PERFECT SCORE X A-PLUS 2013 SEKOLAH BERASRAMA PENUH SBP. Web To explain these observations using Le Chateliers Principle.
The solid dissolves readily in water and its solutions have a salt-like tastePotassium chloride can be obtained from ancient dried lake deposits. All chemical reactions eventually reach a state in which the rate of the reaction in the forward direction is equal to the rate of the reaction in the reverse direction. Students can download the syllabus pdf and go through it to plan their studies.
Web With increasing temperature the reaction rate increases but hydrogen production becomes less favorable thermodynamically since the water gas shift reaction is moderately exothermic. L ə ʃ æ ˈ t ɛ l j eɪ or US. Herein we demonstrate a methodology based on Le Chateliers principle to construct SMSIs by treating MgO-supported gold.
Web Download PDF. This shift in chemical equilibrium can be explained according to Le Chateliers principleOver the temperature range of 6002000 K the equilibrium constant for the. Factors affecting equilibrium- Le Chateliers principle.
So portlandite solubility increases at low temperature. Web Pitting corrosion or pitting is a form of extremely localized corrosion that leads to the random creation of small holes in metal. It is a natural science that covers the elements that make up matter to the compounds made of atoms molecules and ions.
This state results when the forward reaction proceeds at the same rate as the reverse reactionThe. The Le Cha teliers principle states that if a stress such as changing temperature pressure or concentration is inflicted on an equilibrium reaction the reaction will shift to restore the. Their composition structure properties behavior and the changes they undergo during a reaction with other substances.
A lowering of temperature favors the removal of dissolution heat from the system and thus favors dissolution of CaOH 2. Web Besides improving the total oxidation of CO by Fe 3 O 4 following Le Chateliers principle the removal of CO 2 by CaO during CH 4 oxidation has several other benefits. Constant volume and constant pressure depending on how the process is completedThe constant volume.
Web Potassium chloride KCl or potassium salt is a metal halide salt composed of potassium and chlorineIt is odorless and has a white or colorless vitreous crystal appearance. Web Le Chateliers principle pronounced UK. If a chemical reaction is at equilibrium and experiences a change in pressure temperature or concentration of products or reactants the.
Web Chemistry is the scientific study of the properties and behavior of matter. Download Free PDF View PDF. Web Chapter 15 Probability Objective Questions.
Web In a chemical reaction chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time so that there is no observable change in the properties of the system. From the Le Chatelier law alone it is apparent that this exothermic reaction is favored by low temperature and high. Urea serves an important role in the metabolism of nitrogen-containing compounds by animals and is the main nitrogen.
In other words if there is an increase in products the reaction quotient Q_c is increased making it greater than the equilibrium. Web Join an activity with your class and find or create your own quizzes and flashcards. Oilfield scaling is the precipitation and accumulation of.
Scale inhibitors SIs are a class of specialty chemicals that are used to slow or prevent scaling in water systems. Web Urea also known as carbamide is an organic compound with chemical formula CONH 2 2This amide has two amino groups NH 2 joined by a carbonyl functional group CO. Chemistry in Everyday Life.
There are two types adiabatic flame temperature. Web The equilibrium constant K_c defines the relationship among the concentrations of chemical substances involved in a reaction at equilibrium. Web For several decades research groups have attempted to develop iron-based catalysts to direct product selectivity of the FT synthesis toward light olefins 8 9Relative to other FT catalysts such as cobalt iron disfavors competing formation of methane and furthermore catalyzes the water-gas shift reaction enabling the use of a CO-rich syngas.
Cape unit 1 past papers chem. The tip of the arrow points in the direction. Chemistry also addresses the nature.
Web No headers. They consist of chemical or structural formulas of the reactants on the left and those of the products on the right. To relate Le Chateliers Principle to the concept of coupled reactions.
Web In 1905 Fritz Haber 1868-1934 began to study this reaction employing the thinking initiated by Le Chatelier and others and the newly-developing field of thermodynamics that served as the basis of these principles. ICE tables are composed of the concentrations of molecules in solution in different stages of a reaction and are usually used to calculate the K or equilibrium constant expression of a reaction in some instances K may be given and one or more of the concentrations in the table will be the unknown to be solved for. Web CAPE Chemistry Past Paperspdf.
It is thus the simplest amide of carbamic acid. Web They are basically in chronological order subject to the uncertainty of multiprocessing. ˈ ʃ ɑː t əl j eɪ also called Chateliers principle or the Equilibrium Law is a principle of chemistry used to predict the effect of a change in conditions on chemical equilibriaThe principle is named after French chemist Henry Louis Le Chatelier and sometimes also credited to Karl.
Class 10 Science MCQs. It is an upper bound of the temperature that is reached in actual processes. Web Le Ch â telier s principle states that if the system is changed in a way that increases the concentration of one of the reacting species it must favor the reaction in which that species is consumed.
Calcined in 15 vol CO 2 N 2 at 600 C. Equilibrium constant factors affecting equilibrium Le Chateliers principle ionic equilibrium- ionization of acids and bases strong and weak electrolytes degree of ionization. Web Review NEET 2023 syllabus below and download its PDF by using the link of NEET information brochure given below.
The arrow is read as the word yields. Le Cha teliers principle states that if a dynamic equilibrium is disturbed by changing the conditions the position of equilibrium shifts to counteract the change to reestablish an equilibrium.

7 Equilibrium 2021 Docx

Temperature Word Problems Teaching Resources Teachers Pay Teachers

7 Equilibrium 2021 Docx

9 6 Le Chatelier S Principle Pdf Pdf Chemical Equilibrium Chemical Reactions

Le Chatelier S Principle

Applying Le Chatelier S Principle The Contact Process

9 6 Le Chatelier S Principle Pdf Pdf Chemical Equilibrium Chemical Reactions
Le Chatelier S Principle Physics Forums
Le Chatelier S Principle Ppt Download
Chapter 15 Chemical Equilibrium Ppt Download

Le Chatelier Principle Pdf

Soundness Test Of Cement By Le Chatelier Apparatus Bonus Tips Definecivil

7 Equilibrium 2021 Docx

7 1 Le Chatelier S Principle Concentration And Pressure Sl Youtube
15 Types Of Cranes Used In Construction 15 Surprise List Definecivil

Syllabus Et Pdf Vocational Education

Changes In Equilibrium Systems Le Chatelier S Principle The Haber Process Learning Goals I Will Understand Le Chatelier S Principle In Terms Of What Ppt Download